Ryan Sayko, Michael Jacobs, and Andrey V. Dobrynin
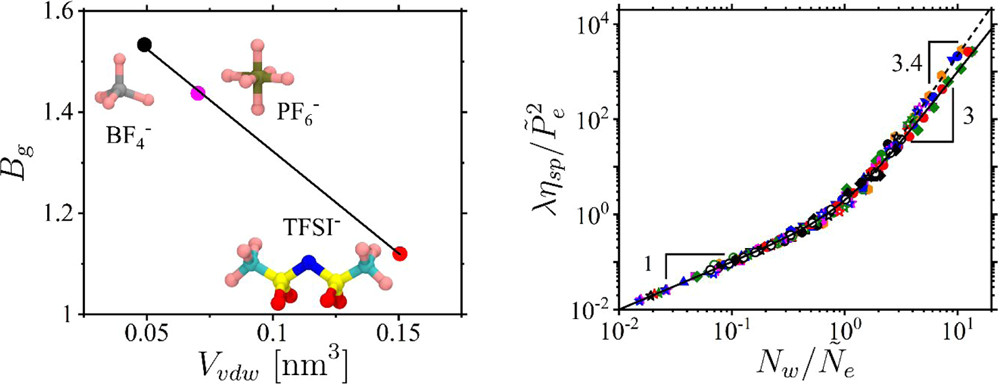
Knowledge of the interaction parameters for polymers in different solvents is crucial for exploring the viscoelastic properties and processing capabilities of polymer solutions. Here, we apply a scaling theory of semidilute polymer solutions to quantify the solution properties of cellulose, poly(ethylene oxide), poly(methyl methacrylate), and poly(acrylonitrile-co-itaconic acid) in different ionic liquids. The starting point of our approach is a scaling relationship between the solution correlation length ξ = lgν/B and the number of repeat units g in a correlation blob for polymers with a repeat unit projection length l, chain segment fractal dimension 1/ν, and the corresponding interaction parameter B describing polymer–solvent affinity and assuming values Bg, Bth, and 1 in different solution regimes. In the framework of this approach, we obtain crossover concentrations to the semidilute solution regime, c*, the solution regime of overlapping thermal blobs, cth, and the concentrated solution regime, c**. The method takes advantage of the linear relationship of the specific viscosity ηsp with the number of correlation blobs per chain Nw/g in the unentangled Rouse regime for polymers with a weight-average degree of polymerization Nw. The application of our approach to entangled solutions provides the chain packing number, Pe, and completes the set of parameters {Bg, Bth, and Pe} that uniquely describes the static and dynamic solution properties for a given polymer/solvent pair. This approach also allows us to identify a trend relating the Bg parameter to the van der Waals volume of the ionic liquid anion, analogous to the Hofmeister series, and to obtain the temperature and solvent type dependence of the polymer excluded volume and Kuhn length.