Connor J. Stubbs, Joshua C. Worch, Hannah Prydderch, Zilu Wang, Robert T. Mathers, Andrey V. Dobrynin, Matthew L. Becker, and Andrew P. Dove
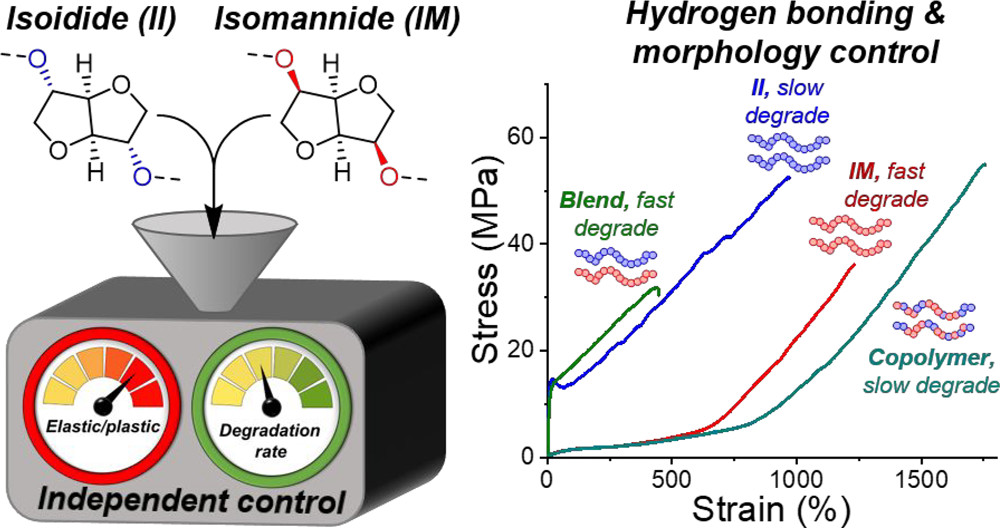
Stereochemistry in polymers can be used as an effective tool to control the mechanical and physical properties of the resulting materials. Typically, though, in synthetic polymers, differences among polymer stereoisomers leads to incremental property variation, i.e., no changes to the baseline plastic or elastic behavior. Here we show that stereochemical differences in sugar-based monomers yield a family of nonsegmented, alternating polyurethanes that can be either strong amorphous thermoplastic elastomers with properties that exceed most cross-linked rubbers or robust, semicrystalline thermoplastics with properties comparable to commercial plastics. The stereochemical differences in the monomers direct distinct intra- and interchain supramolecular hydrogen-bonding interactions in the bulk materials to define their behavior. The chemical similarity among these isohexide-based polymers enables both statistical copolymerization and blending, which each afford independent control over degradability and mechanical properties. The modular molecular design of the polymers provides an opportunity to create a family of materials with divergent properties that possess inherently built degradability and outstanding mechanical performance.